The Employment of Sodium Hydride as a Michael Donor in Palladium‐catalyzed Reductions of α, β‐Unsaturated Carbonyl Compounds - Liu - 2019 - Advanced Synthesis & Catalysis - Wiley Online Library

Complications from dual roles of sodium hydride as a base and as a reducing agent. - Abstract - Europe PMC

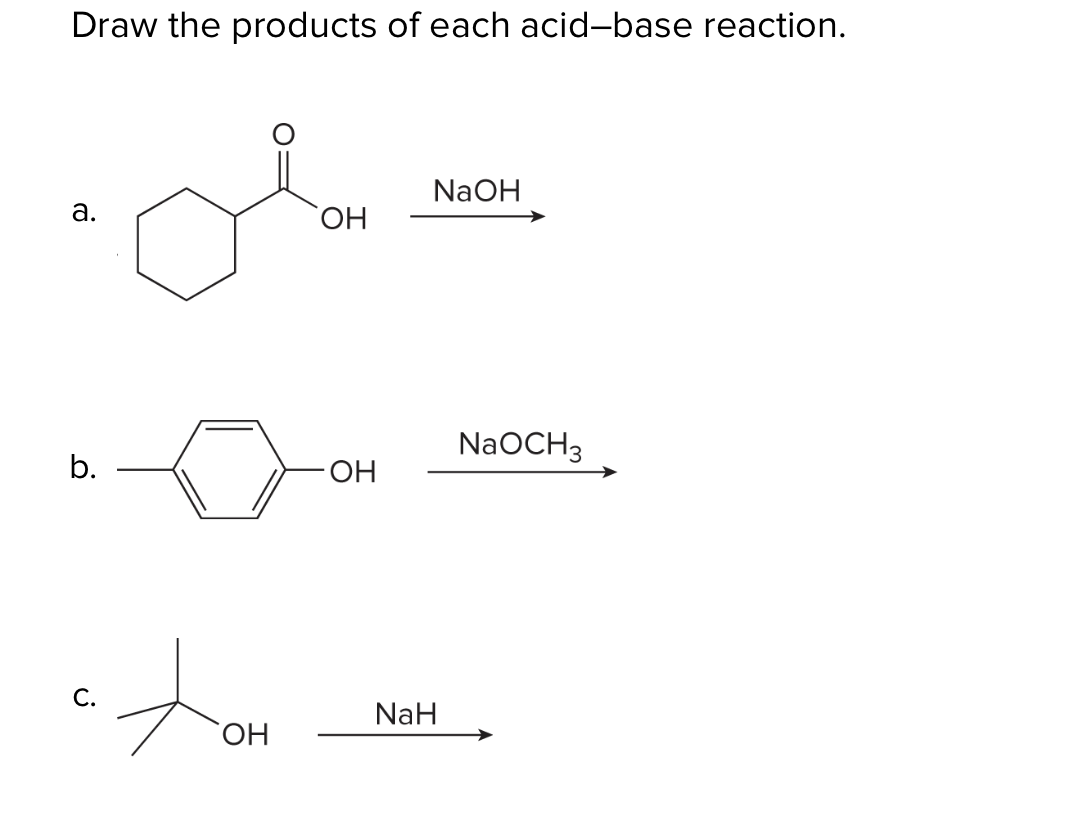
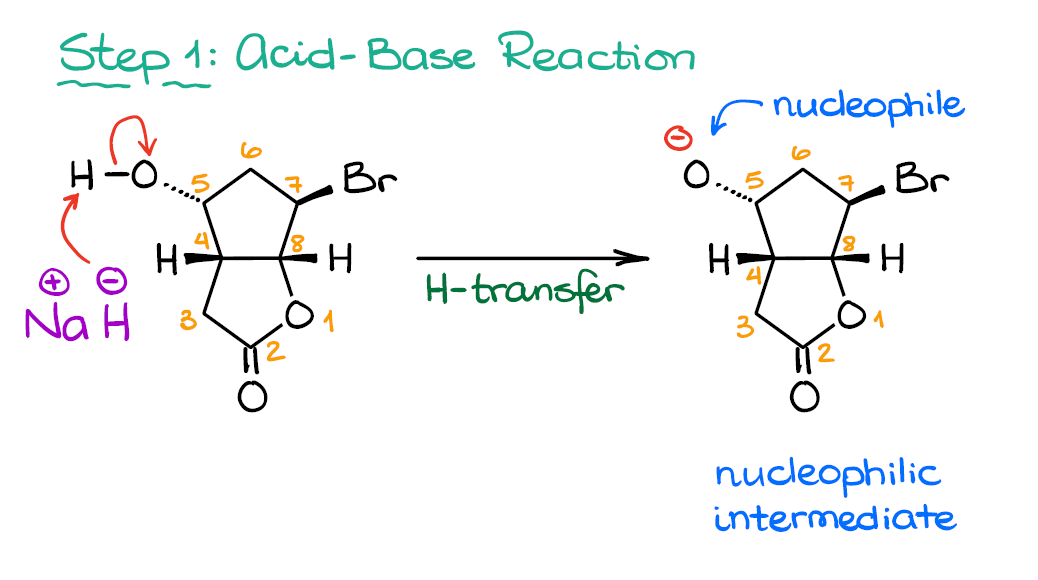
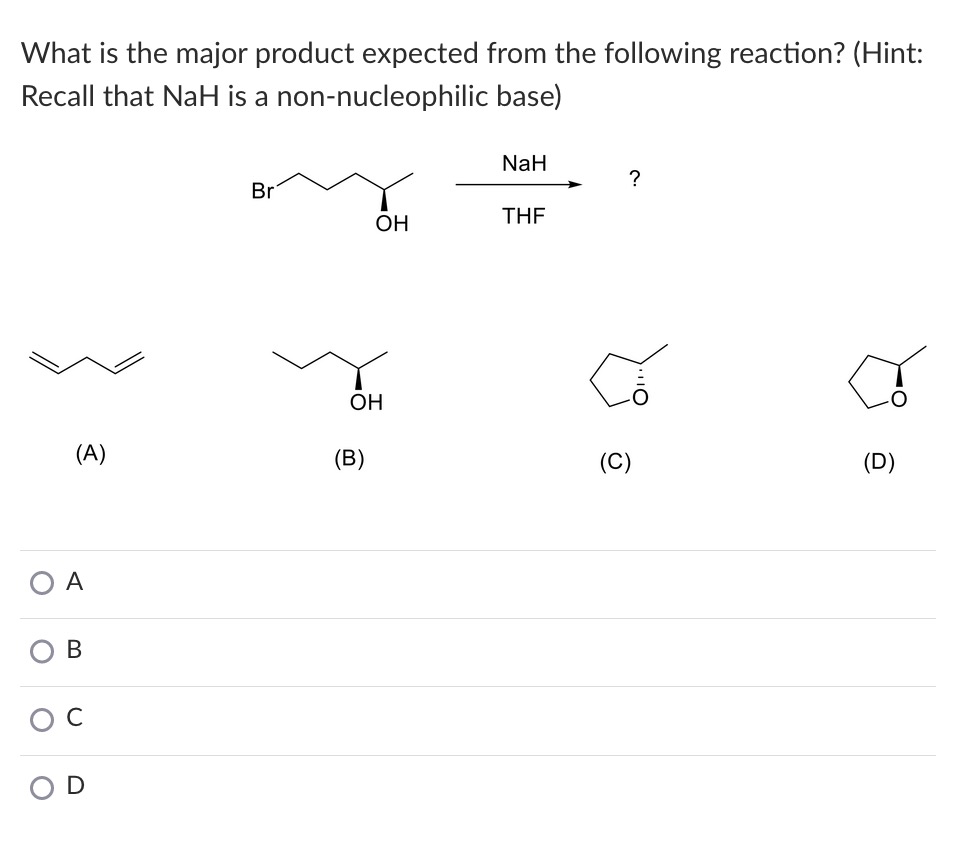
When the halohydrin is treated with NaH, a product of molecular formula C_4H_8O is formed. Draw the structure of the product and indicate its stereochemistry. | Homework.Study.com

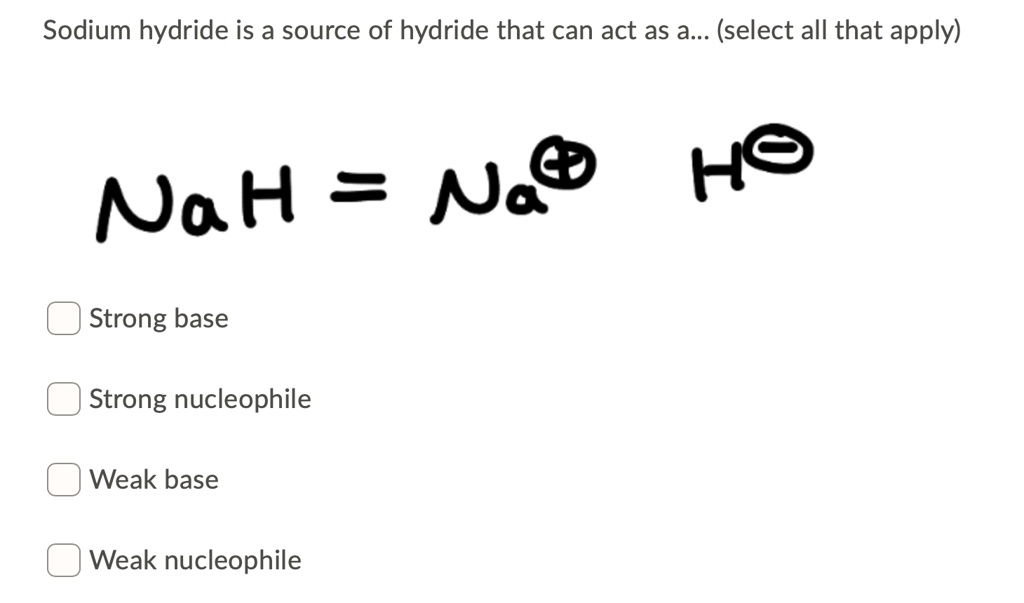
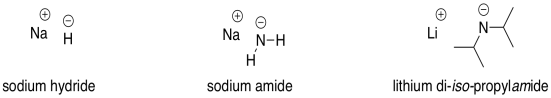
The hydride ion H^(ɵ) is a stronger base than hydroxide ion. Which of the following reaction would occur if NaH is dissolved in water
✓ Solved: When 4-chlorobutane-1-thiol is treated with a strong base such as sodium hydride, NaH, tetrahydrothiophene...

Explosion Hazards of Sodium Hydride in Dimethyl Sulfoxide, N,N-Dimethylformamide, and N,N-Dimethylacetamide | Organic Process Research & Development