
3.16 | Determine the number of moles of compound and the number of moles of each type of atom in - YouTube

Practice Problem How many moles of aluminum oxide will be produced from 0.50 mol of oxygen? 4 Al + 3 O 2 → 2 Al 2 O mol? mol 3 O 2 = 2 Al 2 O ppt download

Chemistry Warm Up: Mole / Mass / Particles 1.What is the mass of one mole of water? 2.If one milliliter of water has a mass of 1.00grams, how many moles. - ppt download

Question Video: Calculating the Mass of Solute Needed to Prepare a Solution with a Desired Concentration and Volume | Nagwa

SOLVED: How many of each the following contained in 100 grams of CO2 (m=44.01)? calculate mol of C,O and O2

Mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 In chemical calculations, mol ratios convert moles of. - ppt download
Objective: Define empirical formula, and explain how the term applies to ionic and molecular compounds


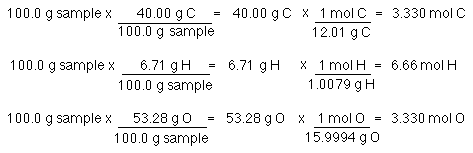
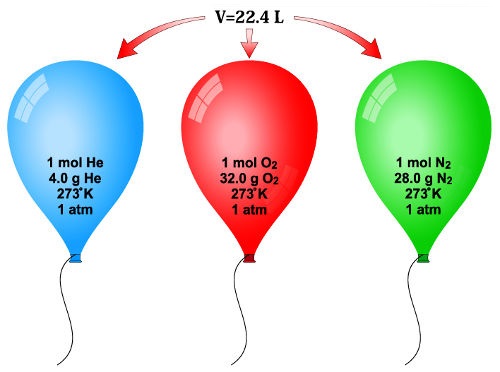


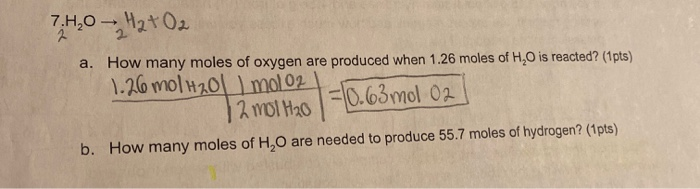

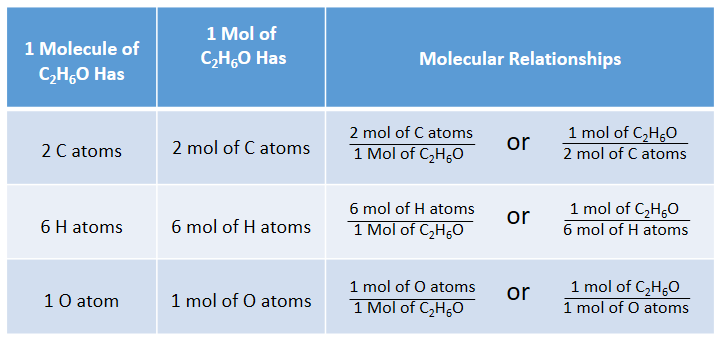
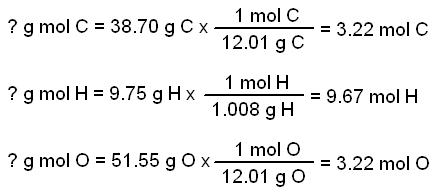



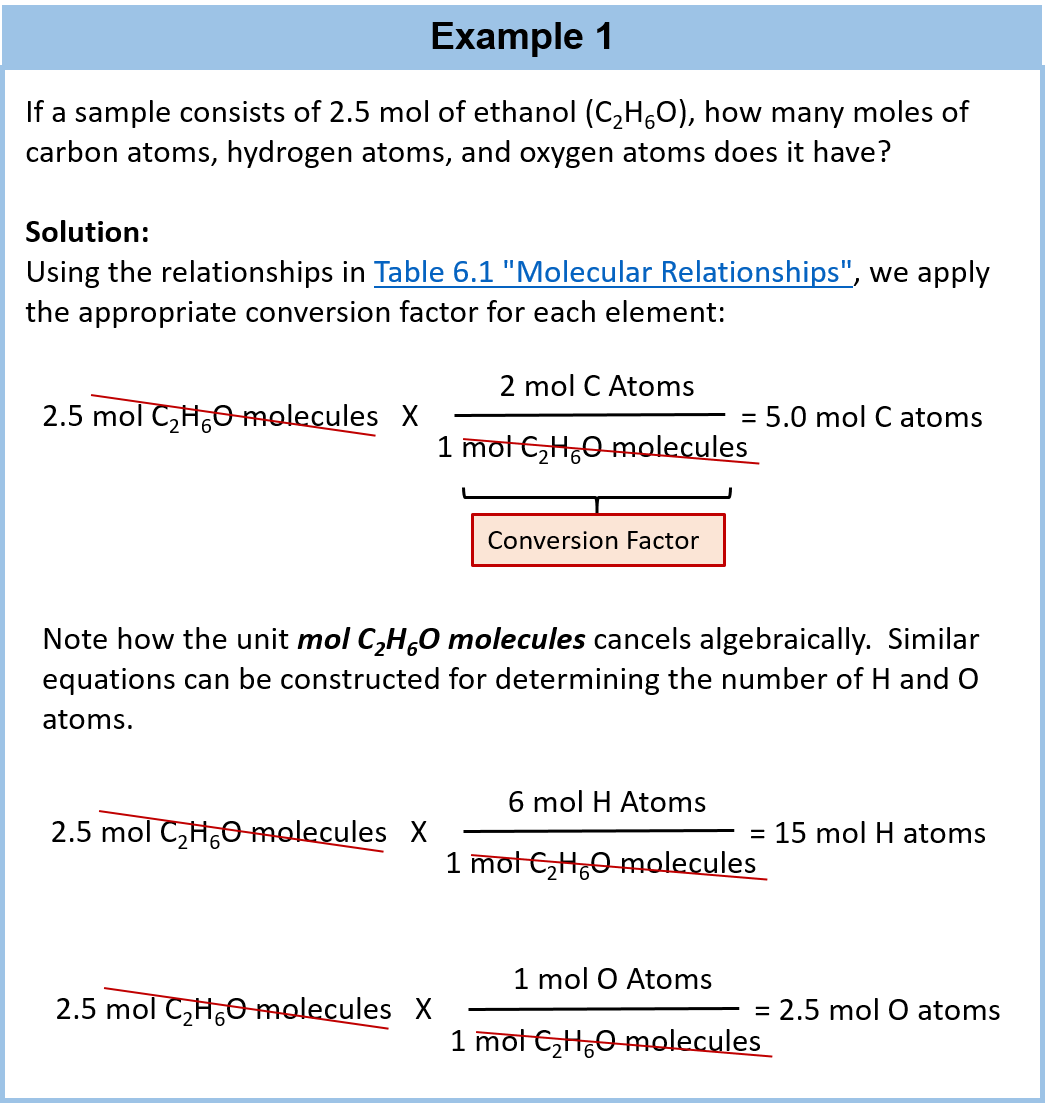
:max_bytes(150000):strip_icc()/pancit-molo-recipe-5209963-hero-01-c89a8ced0df74edb99b4379d413fca58.jpg)
